
Pcl5 Lewis Structure Transborder Media
PCl 5. Back. 70 More Lewis Dot Structures. P does not follow the octet rule. It will hold more than 8 electrons. Phosphorous having valence electrons in the 3rd energy level, will also have access to the 3d sublevel, thus allowing for more than 8 electrons. It will hybridize to form 5 equal dsp3 orbitals and will have a trigonal bipyramidal shape.

Phosphorus pentachloride(PCl₅),formula , molar mass and Lewis dot
Step 1: Count the number of valence electrons in a PCl5 molecule. We can refer to the periodic table for this. We come to understand that PCl5 is made up of Phosphorous and Chlorine. Phosphorus, having atomic number 15, has an electron composition of 2, 8, 5. Therefore, it has 5 electrons in its outermost shell.

Is pcl5 polar or nonpolar 🌈Is PCl5 Polar or Nonpolar? Techiescientist
An explanation of the molecular geometry for the PCl5 (Phosphorous pentachloride) including a description of the PCl5 bond angles. The electron geometry for.

PCl5 lewis structure, molecular geometry, hybridization, bond angle
Phosphorus pentachloride is the chemical compound with the formula PCl 5. It is one of the most important phosphorus chlorides/oxychlorides, others being PCl 3 and POCl 3. PCl 5 finds use as a chlorinating reagent. It is a colourless, water-sensitive solid, although commercial samples can be yellowish and contaminated with hydrogen chloride .

MO Diagram, PCl5 Lewis Structure, Molecular Geometry, and Hybridization
1. The central atom, sulfur, contributes six valence electrons, and each fluorine atom has seven valence electrons, so the Lewis electron structure is. With an expanded valence, that this species is an exception to the octet rule. 2. There are six electron groups around the central atom, each a bonding pair.

PCl5 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
PCl5 Lewis Structure - How to Draw the Lewis Structure for PCl5 Wayne Breslyn 723K subscribers Subscribe Subscribed 167K views 10 years ago A step-by-step explanation of how to draw the.
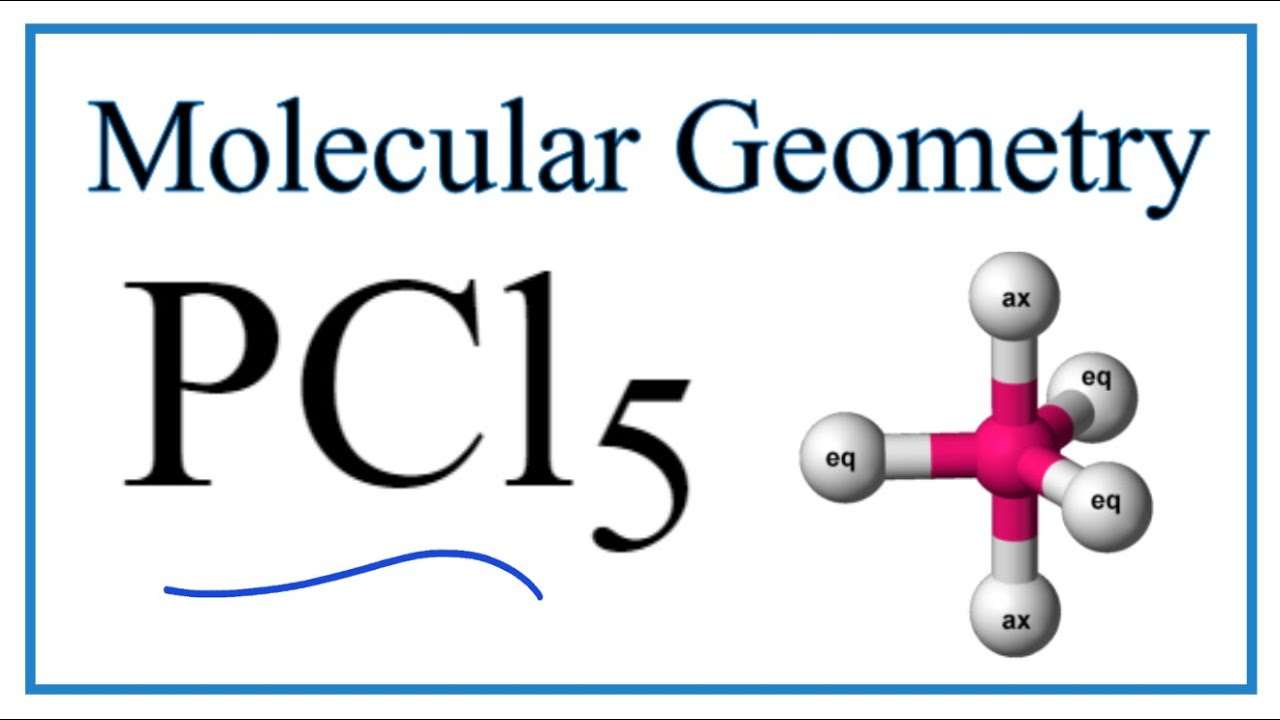
PCl5 (Phosphorus pentachloride) Molecular Geometry, Bond Angles YouTube
-the shape of a molecule. -the reactivity of a molecule and how it might interact with other molecules. -the physical properties of a molecule such as boiling point, surface tension, etc. Drawing the Lewis Structure for PCl 5 Video: Drawing the Lewis Structure for PCl5

Pcl5 Lewis Structure Transborder Media
Check me out: http://www.chemistnate.com
Pcl5 Lewis Structure Octet Rule Draw Easy
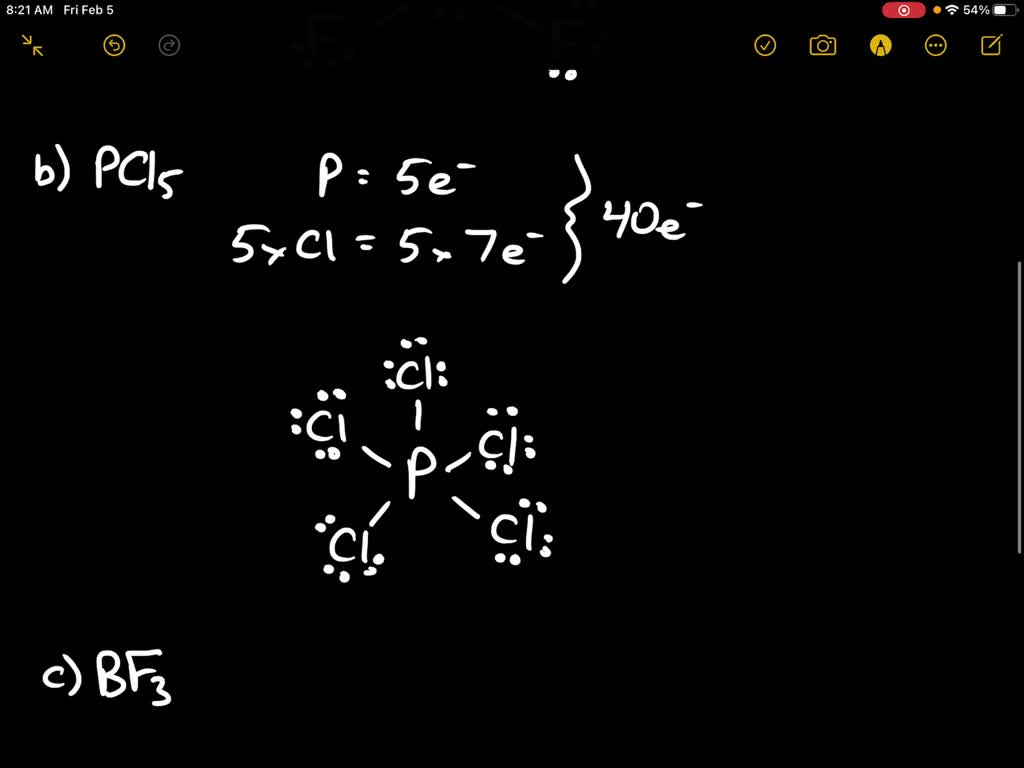
To draw the Lewis structure of PCl5, we follow a series of steps: Determine the total number of valence electrons in PCl5. Phosphorus (P) is in Group 5A and has 5 valence electrons, while each chlorine (Cl) atom contributes 7 valence electrons. Therefore, the total number of valence electrons in PCl5 is 5 + (5 × 7) = 40.

Lewis Structure Pcl5
1. Determine the total number of valence electrons in PCl5. Phosphorus (P) is in Group 5 of the periodic table, so it has 5 valence electrons. Chlorine (Cl) is in Group 7, so it has 7 valence electrons. Since there are five chlorine atoms, the total number of valence electrons is 5 (from P) + 7 (from each Cl) × 5 (number of Cl atoms) = 40. 2.
Gambarkan rumus Lewis dari molekul PCl5
Drawing the Lewis Structure for PCl 5. PCl 5 is similar to PBr 5 and PF 5. If you can do those Lewis structures PCl 5 will be easy. In the PCl 5 Lewis structure Phosphorus (P) is the least electronegative so it goes in the center. In the Lewis structure for PCl 5 there are a total of 40 valence electrons. Five pairs will be used in the chemical.

Draw lewis structures of PCl5 Brainly.in
Steps of drawing PCl5 lewis structure Step 1: Find the total valence electrons in PCl5 molecule. In order to find the total valence electrons in PCl5 (phosphorus pentachloride) molecule, first of all you should know the valence electrons present in phosphorus atom as well as chlorine atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)

Pcl5 Lewis Structure Transborder Media
Step 1: Find valence electrons for all atoms. This is determined by looking at which column on the periodic table the atom is in, ignoring the transition metals in the middle. Add the valence electrons for each atom together. P: 1×5 = 5 Cl: 5×7 = 35 Total = 40 valence electrons Step 2: Find octet electrons for each atom and add them together.

Is PCl5 Polar or Nonpolar? Techiescientist
Find out more about PCl5 in this article on its Lewis Structure, geometry, and hybridization. The chemical formula PCl5 represents the chemical compound Phosphorus Pentachloride. It is a greenish-yellow crystalline solid with an irritating odor.

PCL5 Molecular Geometry,Shape and Bond Angles (Phosphorus Pentachloride
Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron configuration, i.e.
Lewis Structure Pcl5
In this tutorial, we will learn how to draw the lewis structure of PCl 5 step by step with all theories. PCl 5 lewis structure In this lewis structure of PCl 5, center phosphorus atom has made five single bonds with five chlorine atoms. There is a lone pair on center phosphorus atom and each chlorine atom also has three lone pairs.