
Chapter 5.1 Predicting the Geometry of Molecules Chemistry LibreTexts
BrF 5 can be prepared by treating BrF 3 with fluorine under UV light in the region of 300 to 400 nm at room temperature. It was analyzed by UV-Vis, NMR, IR and Raman spectroscopy. Its crystal structure was redetermined by X-ray diffraction, and its space group was corrected to Pnma.

Is SF6 (Sulfur hexafluoride) Polar or NonPolar? YouTube
BrF5 or Bromine Pentafluoride is a polar molecule as the molecular geometry of BrF5 falls out to be square pyramidal with an asymmetric charge distribution concentrating on the central atom. The molecule contains a central bromine atom which is encompassing a total of five fluorides and forming a lone pair of electrons.
PPT Electronegativity & Polarity PowerPoint Presentation, free
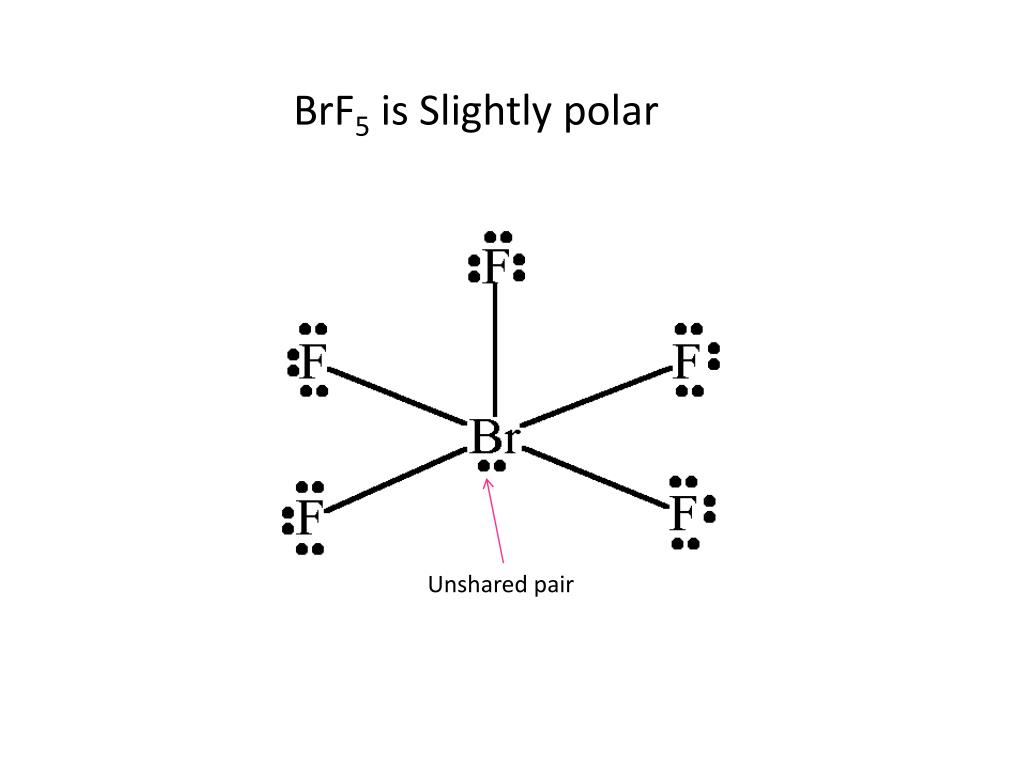
Watch on 0:00 / 2:13 BrF5 comprises Bromine as the central atom with 5 Fluorine atoms surrounding it. It also has two unpaired electrons which att ach themselves to the central atom, Bromine, as a lone pair. Bromine Pentafluoride has a square pyramidal molecular geometry.

BrF5 Molecular Geometry & Bond Angles (Bromine Pentafluoride) YouTube
Bromine pentafluoride (BrF5) is a chemical compound that consists of a central bromine atom bonded to five fluorine atoms. Understanding the Lewis structure of BrF5 is essential in determining its molecular geometry, electron pair geometry, and whether it is polar or nonpolar.
Ch4 Polar Or Nonpolar / Ch4 Polar Or Nonpolar Solved Als CSe2 Polar
Bromine pentafluoride (BrF5), is a polar molecule having a square pyramidal molecular geometry shape and an asymmetric charge distribution centering on the central bromine atom. A central bromine atom encompasses a total of five fluorides and forms a lone pair of electrons in the molecule.

Brf5 Lewis Structure
Bromine Pentafluoride or BrF5 is a fluoride of bromine and an interhalogen compound which means it is made up of only halogen atoms. In the liquid state, it is a colorless, fuming compound having a pungent odor.

Brf3 Polar Or Nonpolar
Bromine Pentafluoride (BrF5) is a polar molecule because the molecular geometry of BrF5 is square pyramidal with an asymmetric charge distribution and with a bong angle of 90°. The molecule has a central bromine atom surrounded by five fluorides and a pair of electrons. Check out the below picture to clarify your confusion further.

MakeTheBrainHappy Is BrF5 Polar or Nonpolar?
Molecular shape or geometry Let's see how each of the above three factors makes bromine pentafluoride (BrF 5) a polar molecule. Factors affecting the polarity of BrF5 Electronegativity Electronegativity is defined as the ability of an atom to attract a shared pair of electrons from a covalent bond. According to Pauling's electronegativity value -
Cf Polar Or Nonpolar Slidesharetrick My XXX Hot Girl
Properties of Bromine pentafluoride It can react with water. It is a powerful oxidizer that can cause severe hazards. It has a molar mass of 174.894 g.mol −1. It has a boiling point of 40.25 °C and a melting point of −61.30 °C. Page Contents show How to draw BrF5 lewis structure

Brf3 Polar Or Nonpolar
Out of all of these, the only polar molecule is $\ce{BrF5}$ (the first one). To review the steps: Draw the Lewis structure; Figure out the geometry (using VSEPR theory) Visualize or draw the geometry; Find the net dipole moment (you don't have to actually do calculations if you can visualize it) If the net dipole moment is zero, it is non-polar.
Is BrF5 Polar or Nonpolar? (Bromine pentafluroide) YouTube
Chemistry Chemistry questions and answers Draw the Lewis structure for BrF5 in the window below and then answer the questions that follow. Is BrF5 polar or nonpolar? _________polarnonpolar This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer

BrF5 Polar or NonPolar Polarity Explained YouTube
Figure 5.3.7: The molecular geometry of a molecule affects its polarity. In CO 2, the two polar bonds cancel each other out, and the result is a nonpolar molecule. Water is polar because its bent shape means that the two polar bonds do not cancel. Some other molecules are shown below (see figure below).

Hexano é Polar Ou Apolar AskSchool
BrF5 is a POLAR molecule because the Br-F bonds present in the molecule are polar and it has asymmetric geometry which causes the partial positive (ẟ+) and partial negative (ẟ-) charge to appear on the molecule. These ẟ+ and ẟ- charges are responsible to make the entire BrF5 molecule polar.

Is BrF5 Polar or Nonpolar? Techiescientist
Bromine pentafluoride, is an interhalogen compound fluoride. It is a strong fluorinating agent BrF finds use in isotope analysis Laser ablation of solid silicates in the presence of BrF releases for subsequent analysis. [2] It has also been tested as an in liquid rocket propellants and is used as a fluorinating agent in the processing of

Polar and Nonpolar Molecules YouTube
Figure 10.2.2 ): (CC BY-NC-SA; anonymous) The two oxygens are double bonded to the sulfur. The oxygens have 2 lone pairs while sulfur had one lone pair. 3. There are two bonding pairs and one lone pair, so the structure is designated as AX 2 E. This designation has a total of three electron pairs, two X and one E.

DOWNLOAD How To Draw The Lewis Dot Structure For Brf5 Bromine
Geometry of Molecules 2.36K subscribers Subscribe 0 110 views 5 months ago Polarity of Molecules Today in this video we are going to determine the polarity of a BrF5 molecule. BrF5 is a.